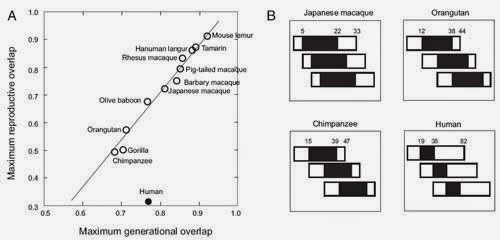
Cant and Johnstones complementing hypothesis that early reproductive cessation reflects “the ghost of
reproductive competition past” (Hrdy 2009 and Cant and Johnstone 2008) predicts
that there will be obvious costs to females that are breeding alongside a
reproductive grandmother. This hypothesis does
not imply that older females who have experienced menopause should not assist
daughters if the dispersal system becomes less female-biased or mothers are
able to maintain kin ties to their daughters.
Variation amongst individuals or
populations in factors that change the intensity or
timing of reproductive competition from the next generation is predicted to associate
with the age at last reproduction as well as reproductive overlap. Social
system variation shown by modern humans also demonstrates the physiological
processes underlying the species-wide trait of rapid reproductive senescence (a cell that is no longer capable of dividing but still alive and metabolically active) compared with the possible flexibility of behavior that
leads to menopause.
The decision to help daughters outside the
family increases the relatedness asymmetry which favours younger females in
within-family conflict, as older females are able to help produce grand offspring
that are related by ¼.
However, assuming that parenthood uncertainty
is extensively accepted as a factor favouring maternal over paternal grand
mothering, it is helpful to compare the magnitude of this effect with the
magnitude of the relatedness asymmetry between older and younger females.
The
kin-selected benefits of helping can explain post reproductive survival, but
not why women cease reproduction so early in the first place. A model
incorporating reproductive competition can help to account for this trait and
for the particular timing of reproductive cessation in human females. Cant and
Johnstone suggest that a combined model that takes into account both the
inclusive fitness benefits of helping and the potential inclusive fitness costs
of reproduction suggestions an improved understanding of the evolution of
menopause.
References:
Hrdy, S.B. (2009). Will the Real Pleistocene Family Please Step Forward? Anon, Mothers and Others: The Evolutionary Origins of Mutual Understanding (pp. 207). United States of America: Anon.
Bereczkei, T., & Dunbar, R.I.M. (1997). Female-biased
reproductive strategies in a Hungarian Gypsy population. Proc R Soc London
Ser B 264:17–22
Johnstone, R.A., & Cant, M.A. (2008). Reproductive conflict and the
separation of reproductive generations in humans, 105(14):5332-5336.
Doi:10.1073/;pnas.0711911105.
Sear R., Mace R., & McGregor I.A. (2000). Maternal grandmothers improve
nutritional status and survival of children in rural Gambia. Proc R Soc
London Ser B 267:1641–1647
Voland E., & Beise J. (2002). Opposite
effects of maternal and paternal grandmothers on infant survival in rural
Krummhörn. Behav Ecol Sociobiol 52:435–443.